PRODUCT CATEGORIES
CLASSES/REGISTRATION
WHAT'S YOUR ROLE?
Updated 2014 Applied Regional Seminars FAQ
There’s a lot going on with oxygen: It’s a drug, its a hazardous material, accreditation has rules, the government has rules and its impossible to keep them all straight!
Applied's experts have created a full day seminar covering how to handle, transport and fill oxygen- as well as other accreditation topics to help home care providers keep up with changing regulatory and technical information.
Applied’s all new 2014 FDA, DOT, OSHA & Accreditation Seminar covers everything you need to meet oxygen training requirements:
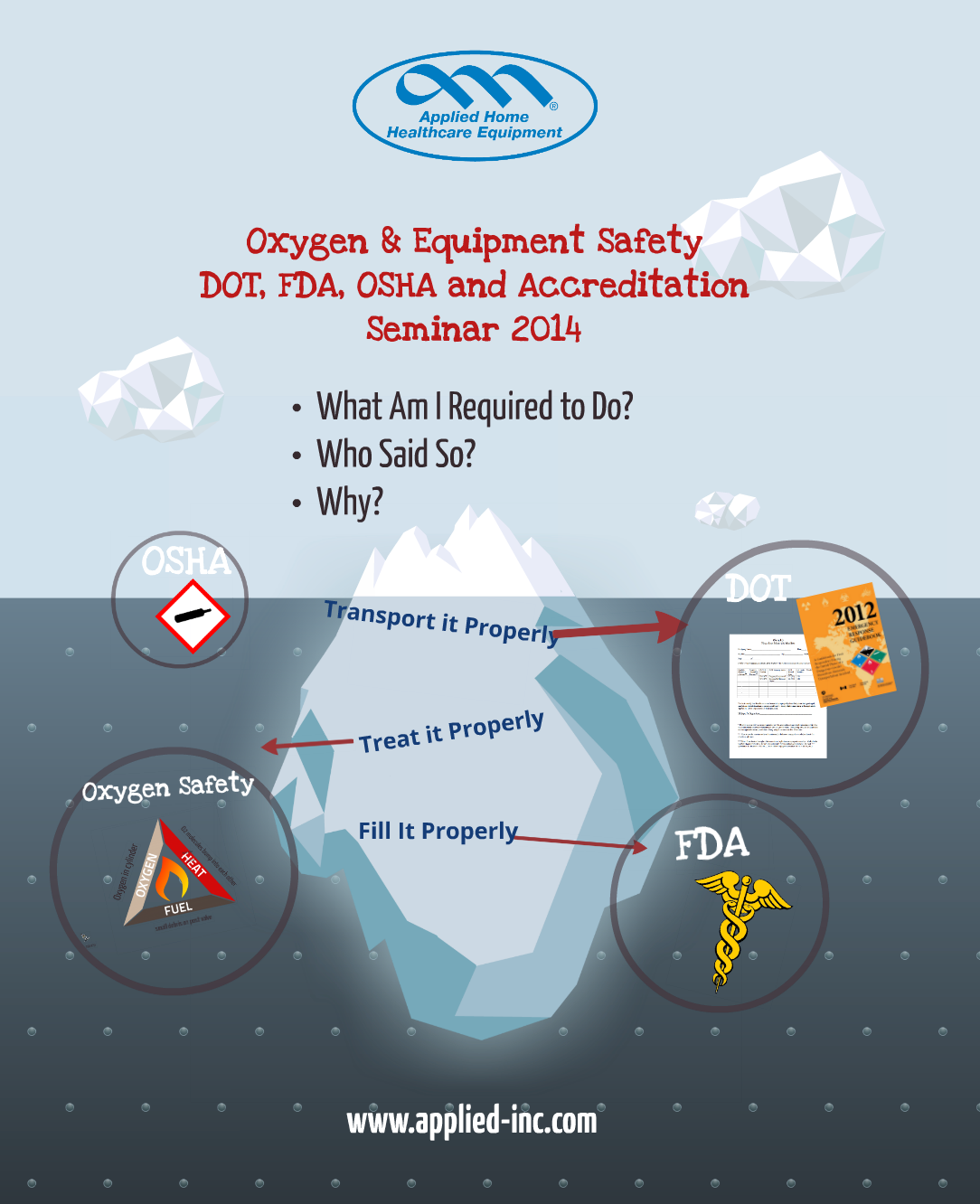 1. Oxygen Safety (Accreditation OSHA, FDA & DOT required)
1. Oxygen Safety (Accreditation OSHA, FDA & DOT required)
Properties of Oxygen
Oxygen Safe Handling
Cylinder Safe Handling
2. Delivery (DOT & Accreditation Required)
Job Specific Training
HazMat Security Awareness Training
HazMat Safety Training
Shipping Papers
Using the NAERG
3. FDA cGMP Training (FDA & Accreditation Required)
Registration
Paperwork
Required Forms
New Labeling Requirements
Filling Procedures
Inspections
4. OSHA
NEW labeling requirements and OSHA SDS training
Applied’s popular presentation has been completely updated with new organization, new information and comes with a book to take home featuring:
All 3 FDA Guidances, DOT interpretations for Homecare Providers, Samples of Shipping Papers, Batch Production Logs, Label Inventory Logs and real warning letters from the FDA to Homecare providers.
How it Works
Our expert comes to a location near you, typically close to an airport, and spends the entire day teaching you in a small class environment- so you can ask questions and get personal attention.
You’ll learn from the experiences of your classmates as well!
Seminars run from 9- 4pm, and include a lunch break with food provided. You’ll take an exam immediately following the instruction, and if you pass (which you will!) you will earn a certificate suitable for training to display your qualifications and satisfy training requirements from the FDA, DOT and Accreditation.
Check out our seminar schedule below. Can't make one of these dates? We've got online and private training options to get you compliant too. Check out our options here.
FDA, DOT & Accreditation Seminar Schedule 2015
Date |
City/State |
Location |
Early Bird Registration |
|---|---|---|---|
|
Tuesday January 13, 2015 9:00am-3:00pm |
Raleigh, NC |
Raleigh Marriott Crabtree Valley 4500 Marriott Drive Raleigh, NC 27612 |
December 15, 2014 |
|
Tuesday |
Philadelphia, PA |
Embassy Suites Philadelphia Int'l Airport 9000 Bartram Avenue Philadelphia, PA 19153 |
January 5, 2015 |
|
Monday |
Las Vegas, NV (Day before Medtrade) |
Mandalay Bay Resort 3950 Las Vegas Blvd. South Las Vegas, NV 89119 |
February 23, 2015 |
|
Tuesday April 14, 2015 9:00am-3:00pm |
Chicago, IL |
Doubletree by Hilton Chicago Oak Brook |
March 16, 2015 |
|
Tuesday April 28, 2015 9:00am-3:00pm |
Cleveland, OH |
Applied Home Healthcare Equipment |
March 27, 2015 |
|
Tuesday |
Nashville, TN |
DoubleTree Suites by Hilton Nashville Airport 2424 Atrium Way Nashville, TN 37214 |
April 13, 2015 |
|
Monday August 17, 2015 9:00am-3:00pm |
Seattle, WA |
DoubleTree by Hilton Seattle Airport |
July 17, 2015 |
|
Tuesday September 15, 2015 9:00am-3:00pm |
Kansas City, MO |
Kansas City Airport Marriott 775 Brasilia Avenue Kansas City, MO 64153 |
August 17, 2015 |
|
Tuesday October 6, 2015 9:00am-3:00pm |
Oklahoma City, OK |
Courtyard Marriott Downtown Oklahoma City 2 West Reno Oklahoma City, OK 73102 |
September 4, 2015 |
|
Monday |
Atlanta, GA (Day before Medtrade) |
Doubletree by Hilton Atlanta Downtown 160 Spring St NW Atlanta, GA 30303 |
September 28, 2015 |
|
Tuesday |
Cleveland, OH |
Applied Home Healthcare Equipment 28825 Ranney Parkway Westlake, OH 44145 |
October 5, 2015 |
Why is this Seminar Required?
Applied's live regional seminar covers topics of training that are required by Accreditation agencies, the US Department of Transportation and the US Food and Drug Administration. Each agency has certain topics that are required to be studied at certain intervals, and this seminar is designed to meet those requirements.
Who Should Take This Seminar?
We recommend that managers, quality control personnel, supervisors, compliance personnel, fillers and delivery drivers.
How Long is the Seminar?
The seminar runs from 9 am to 4pm, with a morning break, lunch break and afternoon break. Instruction usually finishes around 3pm, and the final exam is given and graded in class.
What Do I Do About Lunch?
Lunch is provided! Lunch break is typically from 12-1 and is catered.
Can I Teach Others Once I Take This Seminar?
Yes! This seminar is designed to give you the information to be able to come back to your business and teach others proper DOT, FDA and Accreditation procedures!
What Happens If I Need to Cancel?
If you need cancel, please do so in writing within 10 days of the seminar to receive credit. If you need to cancel less than 10 days out from the seminar, you will be given access to online materials that cover the material. Unfortunately, due to advance class room expenses, we will not be able to refund your registration fee.
What Happens If You Cancel the Seminar?
Applied may cancel the seminar for two reasons: First, if there are not enough participants and second in the event of natural disasters or other emergencies.
If we have to cancel due to lack of attendance, you will be given the option to take the course online or receive a refund for your registration fee.
If we have to cancel due to natural disaster or emergency, we will contact you on the phone number you provided and give you details on how we will make up the seminar. Depending on the scenario, we may re-schedule the seminar or offer it online.
Is there an Online Version of this Seminar?
You can learn the same content online by taking our subscription classes, OF-5000D and OF-5000F.
You Might Also Like
Subscribe to our Email List
Get the latest regulatory info, accreditation news and exclusive discounts!
 View Cart []
View Cart []
